What if I told you that robots smaller than your blood cells could soon be swimming through your veins, hunting down cancer cells with the precision of a heat-seeking missile? It sounds like something straight out of a sci-fi movie, but nanorobotics in medicine isn't just coming – it's already here in early forms.
These microscopic machines are set to transform healthcare in ways we're just beginning to understand. And with the medical nanorobotics market projected to explode into a multi-billion dollar industry, we're looking at one of the biggest shifts in medicine since antibiotics.
What Are These Tiny Medical Robots?
Think of nanobots as the ultimate micro-surgeons. They're engineered devices smaller than human cells that can navigate through your bloodstream like tiny submarines. We're talking about machines that are up to 100 times smaller than your blood cells – so small they can slip between tissues that regular medicine can't reach.
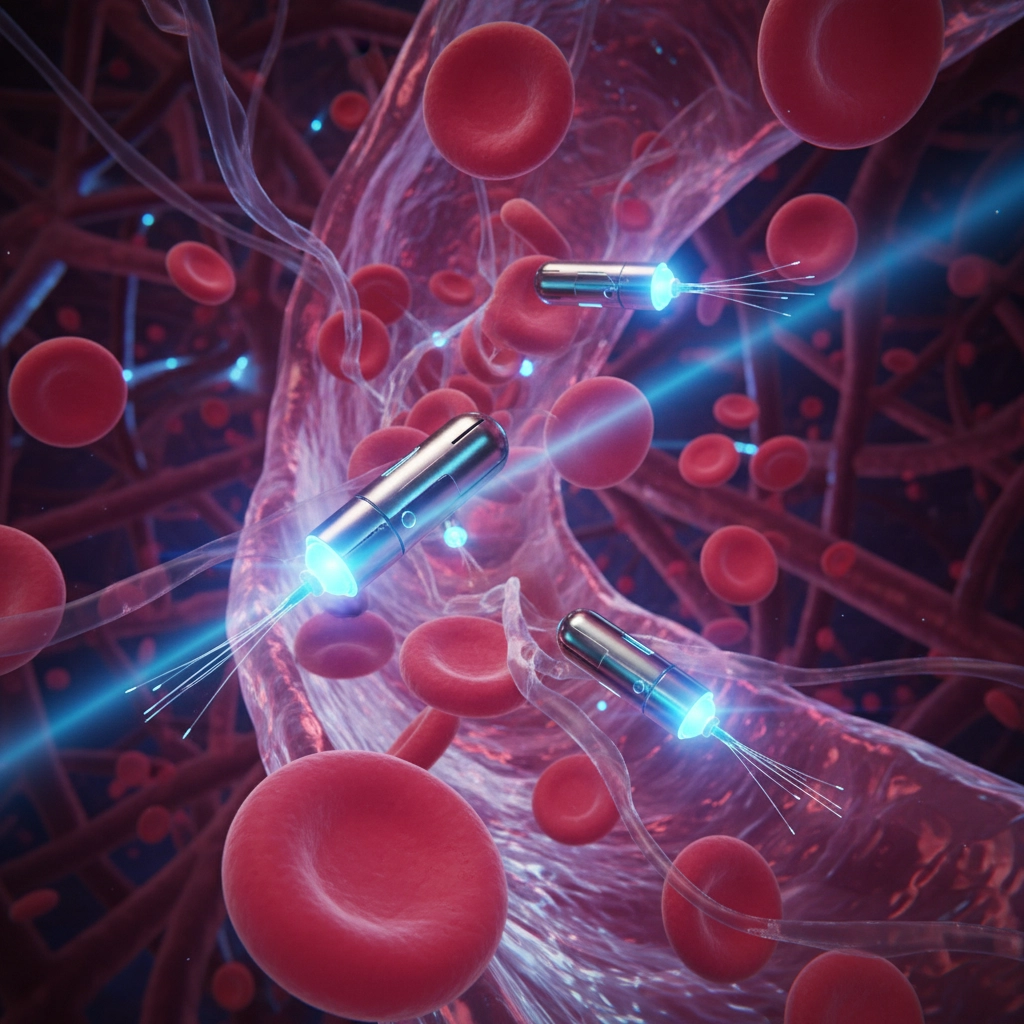
These aren't your typical robots with metal arms and wheels. Medical nanobots are often made from biological materials like DNA strands that can be programmed to recognize specific molecular markers. It's like giving them a wanted poster of exactly which cells to target.
The coolest part? They can carry their own payload. Whether it's chemotherapy drugs, radioactive particles, or clot-inducing medications, these tiny delivery systems can transport treatments directly to where they're needed most.
Cancer Treatment Gets a Major Upgrade
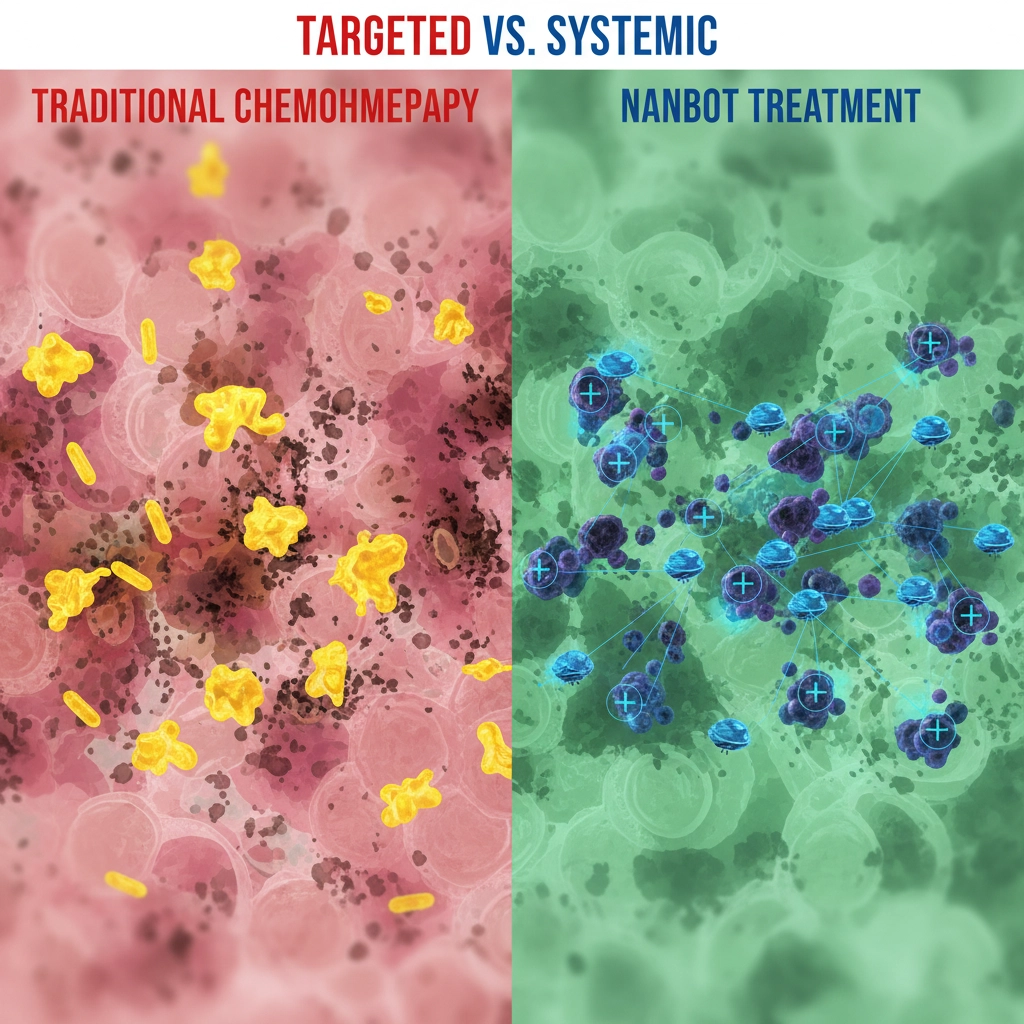
Here's where things get really interesting. Traditional chemotherapy is basically carpet bombing your entire body and hoping it hits the cancer cells. Studies show that up to 99% of chemo drugs never actually reach their intended targets. That's why patients feel so awful during treatment – the medicine is attacking healthy cells too.
Nanobots flip this whole approach on its head. Instead of flooding your system with toxic drugs, these microscopic robots can:
• Target only cancerous cells while leaving healthy tissue alone
• Cross the blood-brain barrier to reach brain tumors
• Cut off blood supply to tumors by delivering clot-inducing drugs to feeding vessels
• Deliver higher doses directly to cancer cells with way fewer side effects
Researchers are already testing DNA-based nanorobots in lab settings. These programmable strands can actually navigate to tumors and essentially starve them by cutting off their nutrient supply. It's like having a special ops team that knows exactly where the enemy is hiding.
Let me paint you a picture of what this could mean. Imagine Sarah, a 45-year-old mom who gets diagnosed with brain cancer. Today, she'd face months of brutal chemo that might not even work because the drugs can't cross into her brain effectively. But in the near future, she could receive an injection of nanobots programmed specifically for her tumor's molecular signature. These tiny robots would swim through her bloodstream, cross into her brain, and deliver targeted therapy right to the cancer cells – all while she goes about her normal day.
Beyond Cancer – Where Else They're Headed
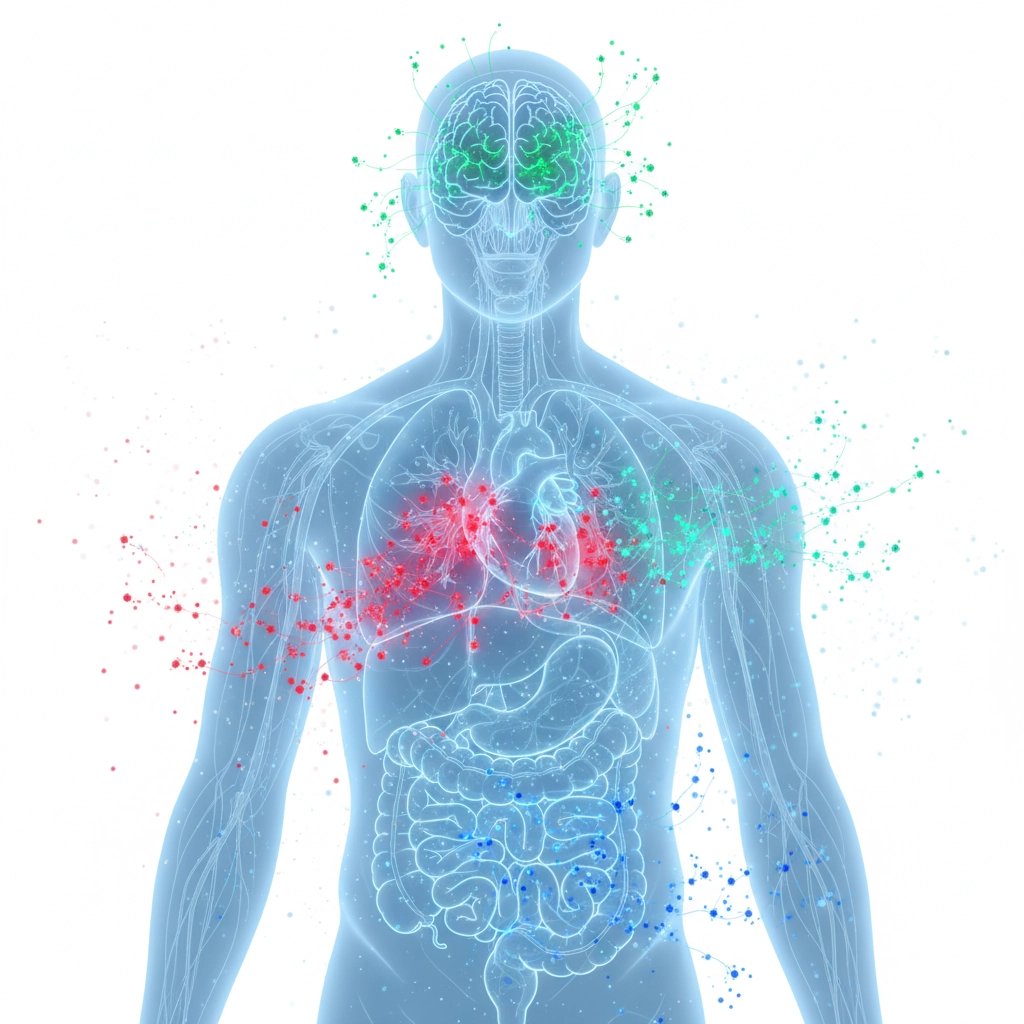
Cancer treatment might be grabbing headlines, but nanobots have their sights set on way more than just tumors. The cardiovascular system is another major target. These microscopic machines could revolutionize how we treat heart disease and stroke.
Picture nanobots designed to patrol your arteries, looking for dangerous clots before they cause a heart attack. Or imagine tiny robots that can perform microsurgery on blocked coronary arteries without you ever needing to go under the knife.
They're also exploring applications in:
- Targeted antibiotic delivery to fight infections
- Real-time monitoring of vital signs from inside your body
- Repairing damaged tissue at the cellular level
- Early disease detection by identifying biomarkers
The diagnostic possibilities alone are mind-blowing. Instead of waiting for symptoms to show up, nanobots could detect diseases like Alzheimer's or Parkinson's years before traditional methods. They could send alerts to your doctor's smartphone the moment they spot trouble brewing in your system.
The Roadblocks We Still Need to Clear
Now, before you get too excited about signing up for nanobot therapy, there are still some pretty significant hurdles to overcome. The biggest challenge? We're still figuring out how to track and control these tiny machines once they're inside the human body.
Unlike regular medications where we just measure the end result, nanobots need to be monitored in real-time. Scientists need to know where they're going, what they're doing, and how they're interacting with different tissues. It's like trying to track a submarine the size of a grain of sand while it's navigating through a maze.
Computer modeling and simulation are helping bridge this gap, but we're still years away from having the complete picture. There are also questions about how the body's immune system will react to these foreign robots, and what happens to the nanobots after they complete their mission.
Safety testing is another massive undertaking. When you're dealing with technology that can cross biological barriers and interact with cells at the molecular level, the margin for error is basically zero. Every component needs to be biocompatible and biodegradable.
Then there's the manufacturing challenge. Creating billions of identical nanobots with precise programming capabilities isn't exactly like rolling cars off an assembly line. The technology exists, but scaling it up for mass production while keeping costs reasonable is still a work in progress.

The regulatory pathway is another maze to navigate. How do you test the safety and efficacy of robots that are too small to see without specialized equipment? The FDA and other regulatory bodies are still developing frameworks for evaluating these technologies.
But here's the thing – despite all these challenges, progress is happening fast. Major pharmaceutical companies and tech giants are pouring billions into nanorobotics research. Universities are graduating more nanotechnology specialists every year. And the potential payoff is so enormous that investment keeps flowing.
Some experts predict we'll see the first FDA-approved medical nanobots within the next decade. Others think it'll take longer. But either way, this technology isn't a question of "if" – it's a question of "when."
What really gets me thinking is how this could change the entire relationship between patients and medicine. Instead of reactive treatment after you get sick, we could shift to a world of continuous, microscopic health monitoring and prevention.
So here's my question for you: if nanobots could patrol your body 24/7, detecting and treating problems before you even knew they existed, would you want them? And what would that mean for how we think about privacy, autonomy, and what it means to be human?






